Well, they can lock us out of The Q Tree, but they can’t stop the truth from getting out.
Enjoy a post OVER HERE.
Here is a quickie in my WAR ON REMDESIVIR.
Fellow Treeper barkerjim dropped an interesting document today, from back in July, which showed the NIH mentioning black sheep IVERMECTIN on the same page as REMDESIVIR.
LINK: https://www.covid19treatmentguidelines.nih.gov/tables/table-2e/
ARCHIVE: https://archive.fo/VNwhF
Such a beautiful misdirection. These guys are MAGICIANS.
This is a perfect example of my postulate that fighting FOR ivermectin will not yield results for restoring real science as fast as fighting AGAINST remdesivir.
In fact, I would go so far as to say that the enemy realized that getting us to fight FOR the saving drug would keep us from expending our energy fighting AGAINST the murdering drug that kills us off and gives them money for doing it.
You may recall my previous posts about remdesivir.
Remdesivir Is How We Bring Down The Temple of Faucism

The Murder of Veronica Wolski by Fauci and Gilead’s Zyklon D

My next piece was going to be an expansion on Karl Denninger’s recent post which places remdesivir/ivermectin and remdesivir/hydroxychloroquine in the context of Anthony Fauci and the disturbingly similar case when he was “all about AIDS” – namely, AZT/bactrim.
LINK: https://market-ticker.org/akcs-www?post=243640
YES. As Cthulhu has said before, “This is not Fauci’s first rodeo.”
Before there were hydroxychloroquine and ivermectin as innocent victims – good Samaritans accused falsely before the world – there was BACTRIM.
And there was FAUCI on all of them. AZT played the murderous part of remdesivir long before we forgot that “miracle drug”.
However, this new information from barkerjim’s drop right here needs to get out right away. The Q Tree site was brought down YET AGAIN as I started working on this, and again when I resumed, so I know it’s critical stuff. The ChiComs have a huge investment – both financial and military 4GW – in the American-killing drug remdesivir. They WILL protect it.
We know from doctors and scientists quoted in my first two articles, that remdesivir has a horrible track record – shocking, really – of renal toxicity. Studies of the drug against Ebola were TERMINATED because it was killing people in the hospital.
How déjà vu.
But here it comes again.

I read the same study results that the above celebratory announcement was made over. Those results were nothing to cheer about, with shot kidneys just the horrifying icing on the death cake. In my opinion, the results were far WORSE than any prior results for hydroxychloroquine. The results – to me – made HCQ look EXCELLENT in comparison.
Yes – by controlling what is acceptable science and what is not, Fauci was able to force the world to field a BAD, DANGEROUS DRUG that made money for Gilead, over a safe, mildly (but critically) effective drug, that made money only for the generics industry, and a French company.
And to top it off, Fauci USED Trump, who could do absolutely nothing about it, to take a KILLER drug into market as the ONLY way to treat his little pandemic.
So let’s take a look at that page dropped by barkerjim. I have captured it as SIX IMAGES.
Again, the link: https://www.covid19treatmentguidelines.nih.gov/tables/table-2e/



As you can see by our comments on The U Tree, most people will look at this table and think they are seeing positive and reasonable behavior by NIH. Adverse events are being discussed, and it appears that things are “even-handed” between different drugs.
And that is EXACTLY the style in which EVIL ABOUNDS IN WASHINGTON, DC (or Atlanta). Good and evil are forced into compromises where GOOD LOSES and EVIL WINS – but the result is called “meeting in the middle”.
CLOSER INSPECTION of the table gives you this, under Adverse Events for remdesivir.
- Nausea
- ALT and AST elevations
- Hypersensitivity
- Increases in prothrombin time
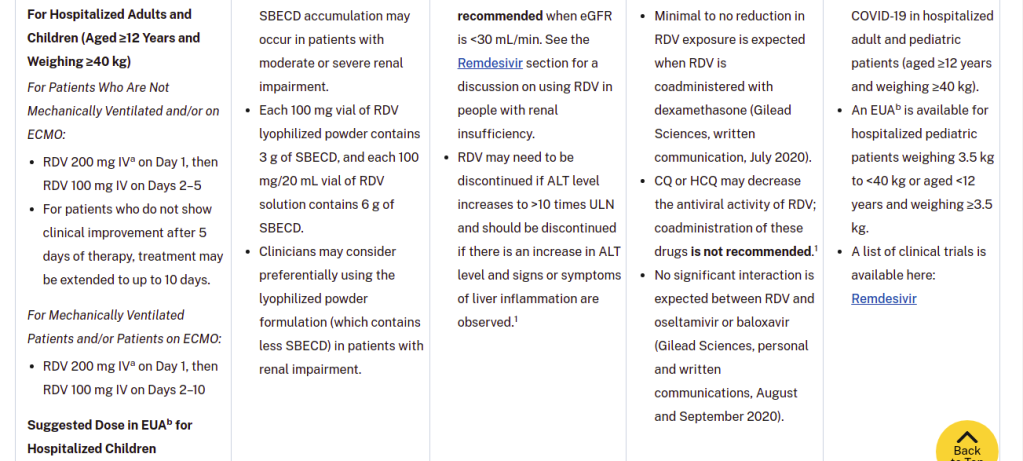
- Drug vehicle is SBECD, which has been associated with renal and liver toxicity. SBECD accumulation may occur in patients with moderate or severe renal impairment.
- Each 100 mg vial of RDV lyophilized powder contains 3 g of SBECD, and each 100 mg/20 mL vial of RDV solution contains 6 g of SBECD.
- Clinicians may consider preferentially using the lyophilized powder formulation (which contains less SBECD) in patients with renal impairment.
This is some of the most remarkable “medical misinformation” I’ve ever seen. It’s truly a work of art.
NIH has HIDDEN – completely hidden – the pronounced renal toxicity of remdesivir. They have hidden it COMPLETELY. It’s GONE. What you are seeing there – the talk about renal and liver toxicity – is a BLAME-SHIFT to a substance that is used WIDELY in intravenous formulations, called sulfobutylether-β-cyclodextrin, or SBECD for short.
This substance is an EXCIPIENT.
An excipient is a substance that is used to MIX with a drug, and take that drug into a form where it can be ADMINISTERED easily. Thus, an excipient may DISSOLVE the drug, or help to dissolve it, into a liquid form. It may help POWDER the drug, so that it can be pressed into tablets or filled into capsules.
Excipients are often considered “inactive ingredients”, even though – YES – they very much can change the effective amount of a drug that the patient gets.
If I had to describe SBECD as something, it would be as a DETERGENT FOR DRUGS. It’s a kind of SOAP made from a cyclodextrin, instead of from some kind of fat or lipid.
And what is a cyclodextrin?

Cyclodextrins are rings of sugar molecules that falls somewhere in between being a smaller chain sugar (like sucrose) and a starch. Cyclodextrins have lots of uses, because they form tubes that act like waffle cones for other molecules. Febreze uses cyclodextrins to trap molecules which have unpleasant odors, at the same time that they release more pleasant ones. A genius application, quite frankly.
Thus, if you make a SOAP that has a little waffle cone for drugs, you can EASILY get drugs to dissolve into a concentrated liquid form by using that soap.
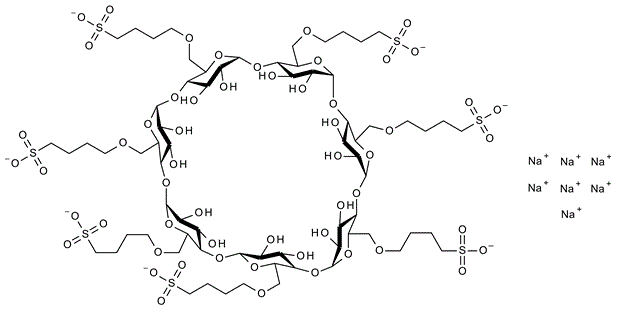
See those sidechains hanging off the cyclodextrin ring? Those are the “SBE” part of SBECD. They are typical of DETERGENTS.
This SBECD stuff and things like it are VERY useful for delivery of drugs. AND they’re relatively safe, too. They are rapidly excreted through the kidneys. Yeah, you don’t want a SOAP piling up in your blood if your kidneys are not working, and THAT is the fact that is being TWISTED by NIH when they say:
Drug vehicle is SBECD, which has been associated with renal and liver toxicity. SBECD accumulation may occur in patients with moderate or severe renal impairment.
Did you catch that sleight of hand? I’m gonna show it to you.
What exactly is causing the renal problems in the FIRST PLACE that you MAY have to be careful about, so that you don’t build up the excipient FOR IT, which MAY constitute a FURTHER risk?
REMDESIVIR.
It’s a crafty little lie. If you have good kidneys, you don’t have anything to worry about with this SBECD crap. But if you have bad kidneys, the LEAST of your problems is SBECD buildup. It’s the remdesivir IN the SBECD that’s gonna kill you.
Weakened kidneys do NOT need to be hit with remdesivir.
Which doesn’t even work ANYWAY. Except to keep people LONGER in the hospital.


Now what you SHOULD be getting, when they administer remdesivir, at the point where the VIRUS is basically gone, and you’re dealing with spike protein damage, cytokine storm, and all that nasty crap, are antiinflammatory, antithrombotic, and immunomodulatory drugs. Even HCQ (a known antirheumatic) at reasonable doses had some antiinflammatory effect in late-stage hospitalized COVID cases, although steroids and other things work better.
When the virus is basically gone, and a bunch of its CRAP is left behind, there is no point administering a toxic antiviral like remdesivir, other than to send money to Gilead Pharmaceuticals and their Deep State friends.
Now, let me stop here and validate this stuff.
HERE is a link that explains how SBECD can be filtered out of blood ANYWAY if a patient has renal impairment.
LINK: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4338618/
Do you see what that means? SBECD is a nothingburger. It’s a DEFLECTION.
The renal problems of remdesivir are never mentioned, by quickly bringing up the risks of the excipient due to the unmentioned damage BY remdesivir.

What NIH did here was to quickly point their finger at THE OTHER GUY and said “HE DID IT!”
This is pure politicized science, where the politics is to defend the drugs and vaccines that enable the shared profits of both the Deep State and the companies that NIH, CDC, and NIAID are in bed with.
Let’s go back to that link I just gave you. THIS part of the conclusions comports very nicely with the reality of SBECD as a widely used excipient.
The finding that SBECD can be effectively removed by CVVH is clinically important, because some cyclodextrins have been associated with hepatotoxicity or nephrotoxicity due to vacuolation [3]. Although our study was small, no evidence to suggest SBECD as a cause of hepatotoxicity or nephrotoxicity was demonstrated in our study patients. This finding is consistent with other SBECD safety studies in humans [3,18]. Additionally, animal studies have only been able to demonstrate cyclodextrin toxicities when dosages more than 50-fold greater (3,000 mg/kg) than those used in humans were administered [3,19,20]. Unlike other cyclodextrins used in these animal studies, SBECD undergoes only minimal tubular reabsorption and limits concentrations within the intracellular tissues of the kidney, potentially reducing the risk of nephrotoxicity. Nevertheless, the FDA labeling for voriconazole recommends that IV therapy be avoided, if possible, in patients with a CrCl <50 ml/min [5]. Our data suggest that IV voriconazole can be safely administered in this population if the patient is concurrently undergoing CVVH.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4338618/
But if you don’t believe THAT study, try THIS ONE.
LINK: https://pubmed.ncbi.nlm.nih.gov/29578585/
Clinical Trial J Clin Pharmacol
2018 Jun; 58(6):814-822. doi: 10.1002/jcph.1077. Epub 2018 Mar 26.
Clinical Pharmacokinetics of Sulfobutylether-β-Cyclodextrin in Patients With Varying Degrees of Renal Impairment
Randall K Hoover 1, Harry Alcorn Jr 2, Laura Lawrence 3, Susan K Paulson 4, Megan Quintas 3, David R Luke 3, Sue K Cammarata 3Affiliations expand
- PMID: 29578585
- PMCID: PMC6718009
- DOI: 10.1002/jcph.1077
Free PMC article
Abstract
Delafloxacin, a fluoroquinolone, has activity against Gram-positive organisms including methicillin-resistant S aureus and fluoroquinolone-susceptible and -resistant Gram-negative organisms. The intravenous formulation of delafloxacin contains the excipient sulfobutylether-β-cyclodextrin (SBECD), which is eliminated by renal filtration. This study examined the pharmacokinetics and safety of SBECD after single intravenous (IV) infusions in subjects with renal impairment. The study was an open-label, parallel-group, crossover study in subjects with normal renal function or mild, moderate, or severe renal impairment, and those with end-stage renal disease undergoing hemodialysis. Subjects received 300 mg delafloxacin IV or placebo IV, containing 2400 mg SBECD, in 2 periods separated by ≥14-day washouts. SBECD total clearance decreased with decreasing renal function, with a corresponding increase in area under the concentration-time curve (AUC0-∞ ). After IV delafloxacin 300 mg administration, SBECD mean total clearance was 6.28 and 1.24 L/h, mean AUC0-∞ was 387 and 2130 h·μg/mL, and mean renal clearance was 5.36 and 1.14 L/h in normal and severe renal subjects, respectively. Similar values were obtained after IV placebo administration. In subjects with end-stage renal disease, delafloxacin 300 mg IV produced mean SBECD AUC0-48 values of 2715 and 7861 h·μg/mL when dosed before and after hemodialysis, respectively. Total SBECD clearance exhibited linear relationships to estimated glomerular filtration rate and creatinine clearance. Single doses of IV delafloxacin 300 mg and IV placebo were well tolerated in all groups. In conclusion, decreasing renal function causes reduced SBECD clearance and increased exposures, but SBECD continues to exhibit a good safety and tolerability profile in IV formulations.
Keywords: Delafloxacin; Hemodialysis; Pharmacokinetics; Renal Dysfunction; Sulfobutylether-β-cyclodextrin.
I’m going to repeat that.
“In conclusion, decreasing renal function causes reduced SBECD clearance and increased exposures, but SBECD continues to exhibit a good safety and tolerability profile in IV formulations.“
Now, the above is not the only “New York Times” style trick that NIH plays here.
Let me list, without going into long-winded explanations, my additional favorites.
- The table authors note that clinical drug-drug interaction studies have not been done, but nonetheless, they say “CQ or HCQ may decrease the antiviral activity of RDV; coadministration of these drugs is not recommended.1” – with a hanging reference.
- For three OTHER potential drug interactions, communications from Gilead are cited as sufficiently exonerating. One is a non-competing generic steroid (dexamethasone) and the other two are patented big pharma antivirals from corporate “frenemy” Genentech. The interaction and “C-level mind-melding” between these two companies is very interesting. Look who just went from one to the other. Interesting times.
- Some crafty shade is thrown at ivermectin by citing a possible adverse event risk and then retracting it, lawyer-style: “Neurological AEs have been reported when IVM has been used to treat parasitic diseases, but it is not clear whether these AEs were caused by IVM or the underlying conditions.” Meanwhile, the DEMONSTRATED risks of remdesivir are not even mentioned.
Bottom line – NIH is protecting Gilead on the toxicity of remdesivir, and they used FAKE NEWS tricks to do it. I keep telling people – science journalism is bad, and science governance is WORSE. It’s been CHINATIZED and OBAMATIZED.
And we’re going to UNDO THAT.
W

Good explainer. I think I have the one liner for those of us in the cheap seats:
Remdesivir, administered with SBECD, damages the kidneys first, which then allows the SBECD to accumulate in the kidneys, thus causing further damage.
Is that right?
Additionally, I have a two word suggestion:
USE IVERMECTIN.
LikeLike
You know the simplest thing about this that just hit me?
I’ll take the medicine that requires 103 words to explain how to use it:
IVERMECTIN.
LikeLike
Very nice explanation of the word games that NIH is playing to get more $$$$ out of a patented death drug.
MORE on
THE FDA USED A FUCKING COMPUTER PROGRAM TO DETERMINE Remdesivir DID NOT HARM THE KIDNEYS!!!
HUMANS ARE THE LAB RATS!!! I am sure PETA is very happy.
LikeLiked by 1 person
What a bunch of SHIT! That is scientific HORSEPOOP.
We KNOW from CLINICAL RESULTS that remdesivir is renally toxic IN VIVO. This is EVASION.
LikeLike
Agreed wolfie,
This may be why those people left the FDA.
I would certainly leave If I was aware of this outright lying that is getting people killed!
LikeLiked by 1 person
People understand that the house of cards is falling. FAUCISM is about to implode.
LikeLike
FAQ sheet for hospitals from Duke Univ (NC): (aka Ass covering)
FAQ: Remdesivir Formulation and Adverse Events
LikeLiked by 1 person
Yeah, this remdesivir stuff is pretty nasty. But IMO they are using it at too high a dose.
The whole point of treating COVID-19 was demonstrated by Didier Raoult (HCQ/AZM), Zelenko (HCQ/AZM/Zn), the ivermectin club, and the Spanish nursing homes (antihistamines). That point is to make the disease non-fatal and non-serious, but nothing more.
This means that viral activity only needs to be reduced by orders of magnitude quickly (Raoult’s data), but does not have to be “cured” by the drug. The point is for the patient to survive.
They need to reduce the dose of remdesivir to the point that it is SAFE TO GIVE IMMEDIATELY and with almost no significant supervision.
ALL they have to do is match the antiviral activity of HCQ or IVM, neither of which is a standard antiviral. This is not hard.
Lower doses of RDV do NOT have to be priced any different. THE CURE IS THE SALE – not the amount of drug.
There is a new study of early remdesivir.
https://www.zerohedge.com/covid-19/gilead-study-shows-remdesivir-reduced-risk-hospitalization-when-given-covid-19-patients
I need to read the whole thing, and the study if I can.
LikeLike
The trouble is, of course, that all one needs to “cure” COVID-19 is a box of antihistamine pills.
Think about that. The fight over vaccines vs remdesivir vs hydroxychloroquine vs ivermectin has all been a lot of crap. Zyrtec gives 100% protection.
OH WAIT, MILLIONS OF PEOPLE WHO TAKE an antihistamine at the slightest sign of a cold already knew this answer. 🙄
LikeLike
Yeah, I was taking Loradadine when I got Covid from UNC. a few weeks later when I went to the ER they gave me Azithromycin and steroid prescriptions and at the ER oxygen and Albuterol to ‘treat wheezing, difficulty breathing, chest tightness, and coughing”
So they KNEW the correct treatment, back in the winter of 2018/2019.
Yeah I had long haulers but the Avermectin cleared that up nicely. It is the height of ragweed season and I am STILL not wheezing and my O2 levels are fine (96% with a nice low pulse in the 60s)
LikeLiked by 1 person
Just so they do not disappear this article:
https://web.archive.org/web/20201230142131/https://scitechdaily.com/pharmaceutical-scientist-warns-of-potential-problems-with-remdesivir-as-covid-19-treatment/
Sci-Tech Daily:
By UNIVERSITY OF CINCINNATI DECEMBER 30, 2020
Pharmaceutical Scientist Warns of Potential Problems With Remdesivir As COVID-19 Treatment
LikeLiked by 1 person
From WIKI
Does Remdesivir help the spike protieins transverse the Blood-Brain Barrier by turning off production of Carboxylesterase 2 ????
LikeLiked by 1 person